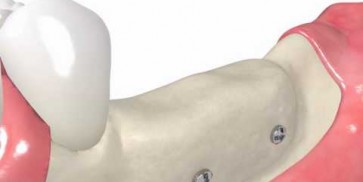
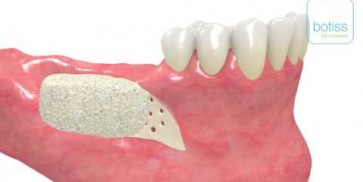
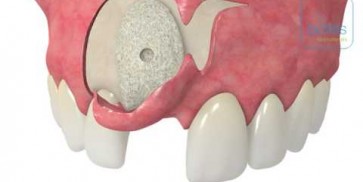
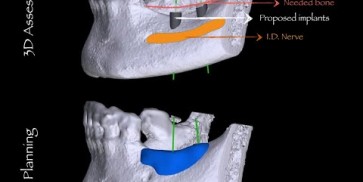
maxgraft® bonebuilder
- Extensive bone defects
- Atrophic maxilla/mandibula
- Horizontal/vertical augmentation
|
- Customized allogenic bone block
- Significantly reduced operation time
Art.-Nr. | Content | |||
|---|---|---|---|---|
PMIa | Individual planning and production of a bone transplant, max. dimensions 23 x 13 x 13 mm | |||
PMIa2 | Additional block(s) for the same patient | |||
32100 | Individual 3D-printed model of the patient’s defect and the planned bonebuilder block (for demonstration purposes) made of plastic | |||

The botiss biomaterials partner Cells+Tissuebank Austria (C+TBA) receives a *.stl milling file and the customized allogenic bone block is produced under clean room conditions. maxgraft® bonebuilder may be applied directly onto the defect. After placement, the maxgraft® bonebuilder block is fixed with osteosynthesis screws. Any residual defect volume should be filled with bone substitute material and the augmentation site covered with a barrier membrane for guided bone regeneration. The individual design provides a precision fit between local bone and the allogenic bone block, enabling rapid revascularization and fast graft incorporation.
Please find our free webinars at www.botiss-webinars.com
Kostenfreie Webinare zu Schulungszwecken finden Sie unter www.botiss-webinars.com
Please find our free webinars at www.botiss-webinars.com
Please find our free webinars at www.botiss-webinars.com
Please find our free webinars at www.botiss-webinars.com
Please find our free webinars at www.botiss-webinars.com
Please find our free webinars at www.botiss-webinars.com
Please Contact us for Literature.